...
The Estate index Si = Ii + Δi of the i-th atom is the sum of its intrinsic state Ii and a perturbation term Δi. The intrinsic state is calculated as

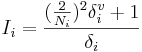 ,
,
where Ni is the principal quantum number for the valence shell of the atom, and 
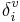 and δi are molecular connectivity delta values. δi = σi − hi equals the number of neighbors in the structure graph, and
and δi are molecular connectivity delta values. δi = σi − hi equals the number of neighbors in the structure graph, and 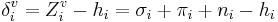 . Here, σi is the number of electrons in σ orbitals, hi is the number of bonded hydrogens,
. Here, σi is the number of electrons in σ orbitals, hi is the number of bonded hydrogens, 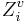 is the number of valence electrons, πi is the number of electrons in π orbitals, and ni is the number of electrons in lone pairs. The difference δv − δ is proportional to valence state electronegativity. The perturbation state is a sum over the intrinsic states of the atoms neighbors, weighted by their squared topological distance:
is the number of valence electrons, πi is the number of electrons in π orbitals, and ni is the number of electrons in lone pairs. The difference δv − δ is proportional to valence state electronegativity. The perturbation state is a sum over the intrinsic states of the atoms neighbors, weighted by their squared topological distance:
...