Summary
Electro-topological state indices combine electronic and graph-topological information about a compound. They were introduced by Lowel H. Hall and Lemont B. Kier in the 1990s.
Theory
The Estate index Si = Ii + Δi of the i-th atom is the sum of its intrinsic state Ii and a perturbation term Δi. The intrinsic state is calculated as
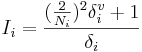 ,
,
where Ni is the principal quantum number for the valence shell of the atom, and 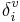 and δi are molecular connectivity delta values. δi = σi − hi equals the number of neighbors in the structure graph, and
and δi are molecular connectivity delta values. δi = σi − hi equals the number of neighbors in the structure graph, and 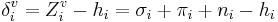 . Here, σi is the number of electrons in σ orbitals, hi is the number of bonded hydrogens,
. Here, σi is the number of electrons in σ orbitals, hi is the number of bonded hydrogens, 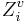 is the number of valence electrons, πi is the number of electrons in π orbitals, and ni is the number of electrons in lone pairs. The difference δv − δ is proportional to valence state electronegativity. The perturbation state is a sum over the intrinsic states of the atoms neighbors, weighted by their squared topological distance:
is the number of valence electrons, πi is the number of electrons in π orbitals, and ni is the number of electrons in lone pairs. The difference δv − δ is proportional to valence state electronegativity. The perturbation state is a sum over the intrinsic states of the atoms neighbors, weighted by their squared topological distance:
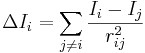 .
.
Estate indices have later been extended to hydrogen atoms and bonds.
Descriptors
EState indices are separated on atom/bond type. In addition to indices, it is also possible to select E-state counts, which correspond to counts of atom or bond types according to the respective indices.
EState indices encode electronic and topological information, and have proven useful in the establishment of QSAR and QSPR models.
Options
Aromatize structures -- Whether to aromatize compounds, and which aromatization method to use.
Atom indices -- Whether to calculate atom e-state indices (intrinsic + perturbation term).
Bond indices -- Whether to calculate bond e-state indices (intrinsic + perturbation term).
Atom counts -- Whether to include counts of atom types.
Bond counts -- Whether to include counts of bond types.
Reference
- Lowell H. Hall, Lemont B. Kier: An atom-centered index for drug QSAR models. In Bernard Testa (editor): Advance in Drug Design, vol. 22, Academic Press, 1992.
- Lowell H. Hall, Lemont B. Kier: Electrotopological state indices for atom types: A novel combination of electronic, topological, and valence state information, Journal of Chemical Information and Computer Sciences 35(6): 1039-1045, American Chemical Society, 1995. ArticleID Q5388.
- Lowell H. Hall, Lemont B. Kier, Briscoe B. Brown: Molecular similarity based on novel atom-type electrotopological state indices, Journal of Chemical Information and Computer Sciences 35(6): 1074-1080, American Chemical Society, 1995.
- Lowell H. Hall, Lemont B. Kier: Molecular Structure Description: The Electrotopological State, Academic Press, 1999.
See this page for an extensive bibliography on Estate indices.