Goal:
During the tutorial, we will learn how to screen chemical libraries against toxicological alerts, to identify potentially hazardous compounds and to interpret the results. For accessing this handout and other related materials, please visit http://docs.eadmet.com/display/EWS2M/
Motivation:
Datasets:
For speed and convenience, we will not use any external SD-files. We have already uploaded the necessary datasets into OCHEM.
In this tutorial we will learn how to browse alerts, create alert sets, screen chemical libraries against the desired alerts and analyze the results.
Datasets.
We will use the "High performance volume" dataset. The whole dataset has been split into "HPV low mol. Weight" and "HPV high mol. weight" according to the molecular weight of the structures. Please, use any of the datasets.
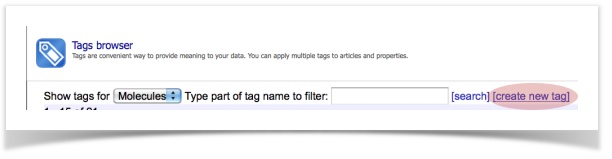
1. Choosing the desired alerts
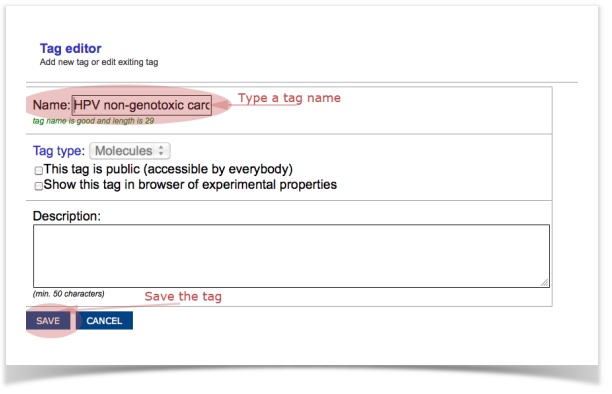
We would like to screen our molecules against five endpoints: acute aquatic toxicity, skin sensitization, non-genotoxic carcinogenicity, genotoxic carcinogenicity and idiosyncratic toxicity.
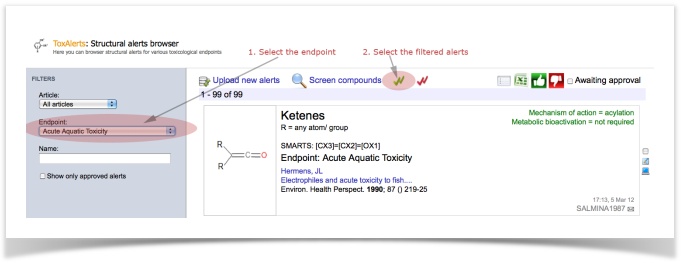
Now, we are ready to screen molecules against the selected alerts!
2. Screening molecules against alerts
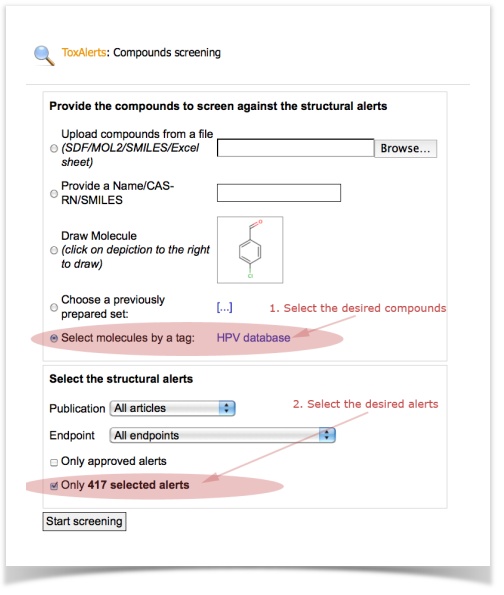
3. Analyze the screening results
The screening results dialog shows the potentially hazardous compounds grouped by endpoints, by alerts and by publications. Please, spend some time to click around.
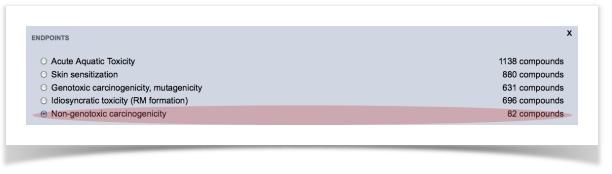
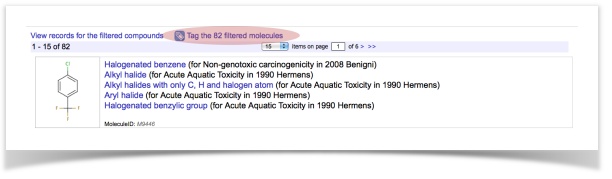
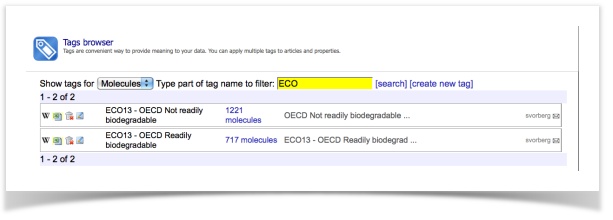
In this tutorial, we will use a dataset of ready-biodegradability. We have prepared two sets of molecules containing (a) 717 readily biodegradable compounds and (b) 1221 non-readily biodegradable compounds.
The datasets were taken from the study of Vorberg et. Al "Modeling the biodegradability of chemical compounds using the On-line CHEmical modeling Environment (OCHEM)", J. Mol. Inf, in review.
| Question of this tutorial: What are the key structural features distinguishing readily biodegradable compounds from non-readily biodegradable ones? |
Follow the steps below:
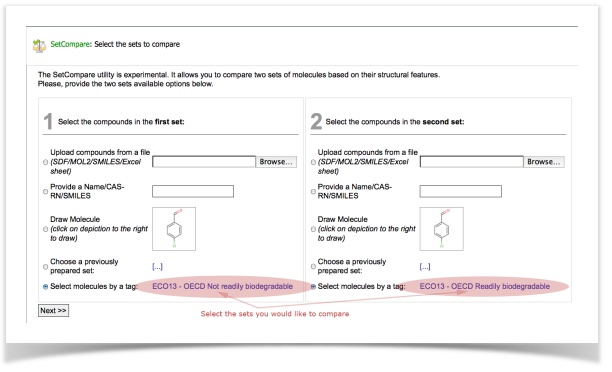
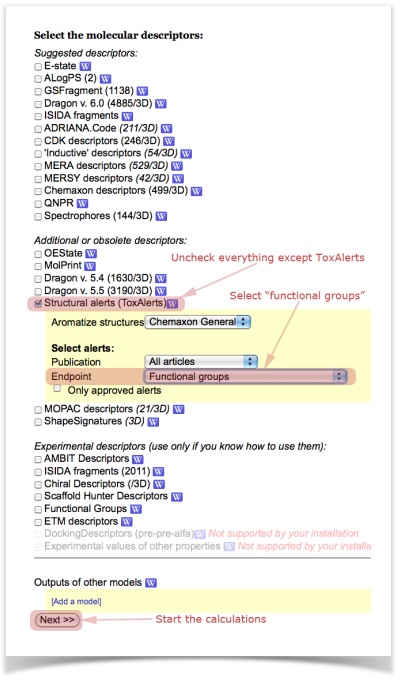
Finally, when your calculation is completed, review and analyze the results!
The screen with results will show you the structural features that are statistically over-represented in the one or the other set.
This exemplary study shows you, for example, that Halogens are over-represented in non-readily biodegradable compounds.
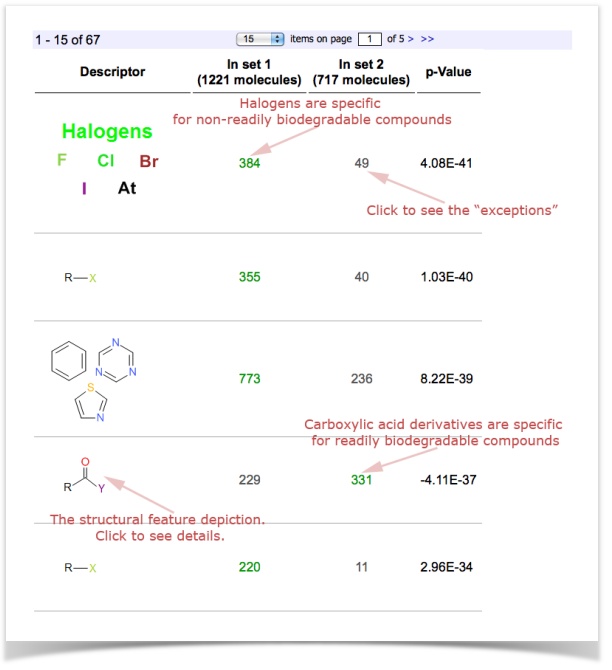
| How to interpret these results? This is up to you as a researcher! |
The tutorials demonstrate a fundamental difference of ToxAlerts (and the alerts-based analysis) from traditional QSAR: interpretability of the former approach.
While QSAR approaches are, in general, more powerful, universal and accurate, the alerts-based approach allows to obtain more interpretable results.
Thus, alerts-based analysis is a useful utility in the toolbox of a chemoinformatician that can complement the conventional QSAR techniques.